Lab Prof. Korbmacher
![]() Member of the Transregio (SFB) TRR374
Member of the Transregio (SFB) TRR374
Research project A4: Tubular and interstitial proteases as regulators of the epithelial sodium channel (ENaC).
Renal epithelial ion channels
Publications of Christoph Korbmacher
The research focus of the group of Prof. Dr. C. Korbmacher is the physiology and pathophysiology of renal and epithelial ion channels. In the kidney and other epithelial organs, ion channels are involved in mediating highly selective and regulated transepithelial ion transport. To study these ion channels and their regulation is of physiological and pathophysiological relevance, because an inappropriate function of renal ion channels may cause for example arterial hypertension, renal salt wasting syndromes, or polycystic kidney disease.
Renal sodium and potassium homeostasis
Sodium homeostasis and potassium homeostasis are intimately linked and critically important for the survival of the human organism. Homeostatic regulation primarily depends on the ability of the kidney to match dietary sodium and potassium intake with appropriate renal excretion. Maintaining sodium balance is essential for the control of extracellular fluid volume and blood pressure. Inappropriate renal sodium retention will cause expansion of extracellular fluid volume and may result in arterial hypertension and edema. In contrast, renal sodium wasting causes extracellular volume depletion resulting in a decrease of arterial blood pressure and eventually circulatory collapse. Maintaining potassium balance is critically important for many cellular functions, including neuronal and cardiac excitability. Renal potassium retention or wasting will ultimately lead to hyperkalemia or hypokalemia, respectively, which may cause cardiac arrhythmias and cardiac arrest. Thus, pathophysiological disturbances of renal sodium or potassium homeostasis result in potentially life threatening disorders. Therefore, it is of great interest to understand the function and regulation of ion channels involved in renal sodium and potassium handling.
Epithelial sodium channel (ENaC)
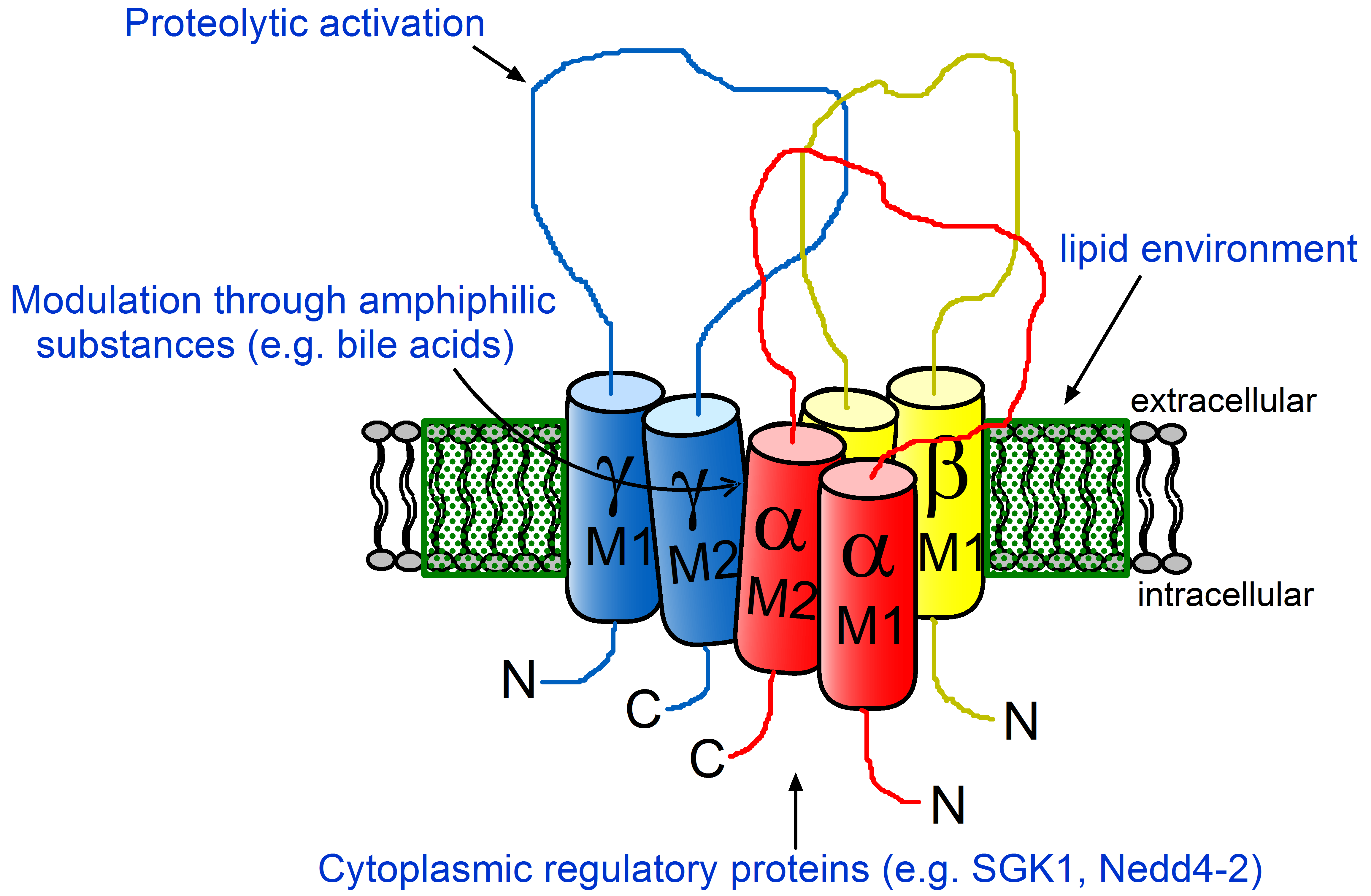
A particular focus of this research group is the amiloride-sensitive epithelial sodium channel (ENaC) and the molecular mechanisms involved in its regulation. Ion flux through ENaC is the rate-limiting step for sodium absorption in the so-called aldosterone sensitive distal nephron (ASDN). The pathophysiological importance of ENaC for sodium homeostasis and blood pressure control is evidenced by ‘gain of function’ and ‘loss of function’ mutations of the channel causing a hereditary form of severe salt-sensitive arterial hypertension (Liddle syndrome; pseudohyperaldosteronism) or a renal salt wasting syndrome (PHA1; pseudhypoaldosteronism type 1), respectively. ENaC also plays an important physiological and pathophysiological role in sodium and fluid absorption by the respiratory epithelium and distal colon.
Regulation of ENaC by hormonal and local factors
A complex network of hormonal and local factors contributes to regulating ENaC. The most important hormone stimulating channel activity is aldosterone which acts through the mineralocorticoid receptor (MR). Many questions remain open regarding regional differences of the action of aldosterone in the ASDN and the molecular mechanisms involved in mediating the aldosterone effect. The differential regulation of sodium absorption and potassium secretion by aldosterone in the ASDN is also incompletely understood. In the ASDN, the secretory potassium channel ROMK (renal outer medullary K+ channel) is mainly responsible for potassium secretion. An increased ENaC activity favors potassium secretion through ROMK. In contrast, inhibiting ENaC, e.g. by amiloride, reduces ROMK mediated potassium secretion. Therefore, the regulatory interplay of the two channels is of great importance for renal sodium and potassium homeostasis. The appropriate adjustment of the functional interaction of ENaC and ROMK is likely to involve a regional heterogeneity of channel regulation. At the cellular and molecular level, several regulatory proteins (e.g. kinases, proteases, and proteins directly associated with the channel) and the lipid environment of ENaC contribute to its regulation.
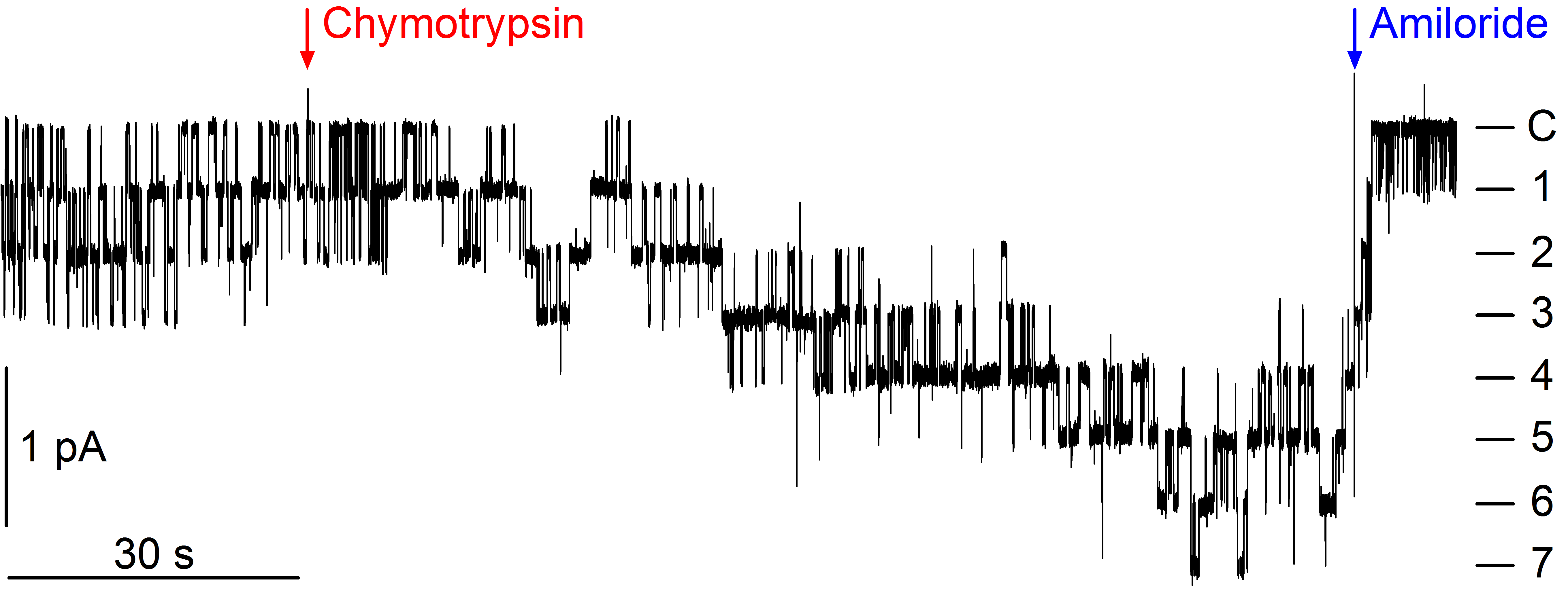
Activation of ENaC by proteases
A specific feature of ENaC is its complex proteolytic processing which is critical for channel activation. Proteolytic channel activation can be nicely demonstrated in heterologous expression systems. ENaC activation by locally released proteases may be pathophysiologically relevant in the context of inflammatory kidney disease and may contribute to sodium retention for example in nephrotic syndrome. However, molecular mechanisms contributing to proteolytic ENaC activation are still incompletely understood and (patho-)physiologically relevant proteases remain to be identified. In addition to proteases activating ENaC directly by proteolytic channel cleavage at specific sites, interstitial proteases may indirectly modulate ENaC mediated transepithelial sodium transport by activating a basolateral protease-activated receptor type 2 (PAR2).
Methods used to study epithelial ion channels
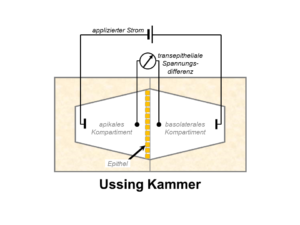
Above all, electrophysiological methods are used to study the function and regulation of renal and epithelial ion channels. These include transepihelial short circuit current measurements in Ussing chambers, whole-cell current recordings using the two-electrode voltage clamp (TEVC) technique, and patch-clamp experiments which in addition to whole-cell recordings also allow single-channel recordings. To elucidate the molecular mechanisms involved in channel regulation, a range of additional molecular biological and cell physiological methods are employed including the use of Xenopus laevis oocytes, cultured cells, native tissue, and animal models (e.g. genetically modified mouse lines). Moreover, the now available structural information in combination with computer simulations and site-directed mutagenesis allows the investigation of functionally relevant channel regions. This integrated approach provides fascinating opportunities to gain novel insights into physiological and pathophysiological mechanisms and a better understanding of molecular disease processes.




